




| MODEL | NOBLE (AP-2021A) |
Filteration System | HEPA Filter System |
Sistem Filter Air | 1st : 4D Pre-Filter 2nd : Air Matching Filter 3rd : Double HEPA Filter 4th : Deodorisation Filter |
| Siklus Penggantian Yang Diharapkan | 1st : - 2nd : 4 Bulan 3rd : 12 Bulan 4th : 24 Bulan |
Air Purifying (Rated Application Floor Area) | 66 m2 |
| Rentang Daya | 55 W |
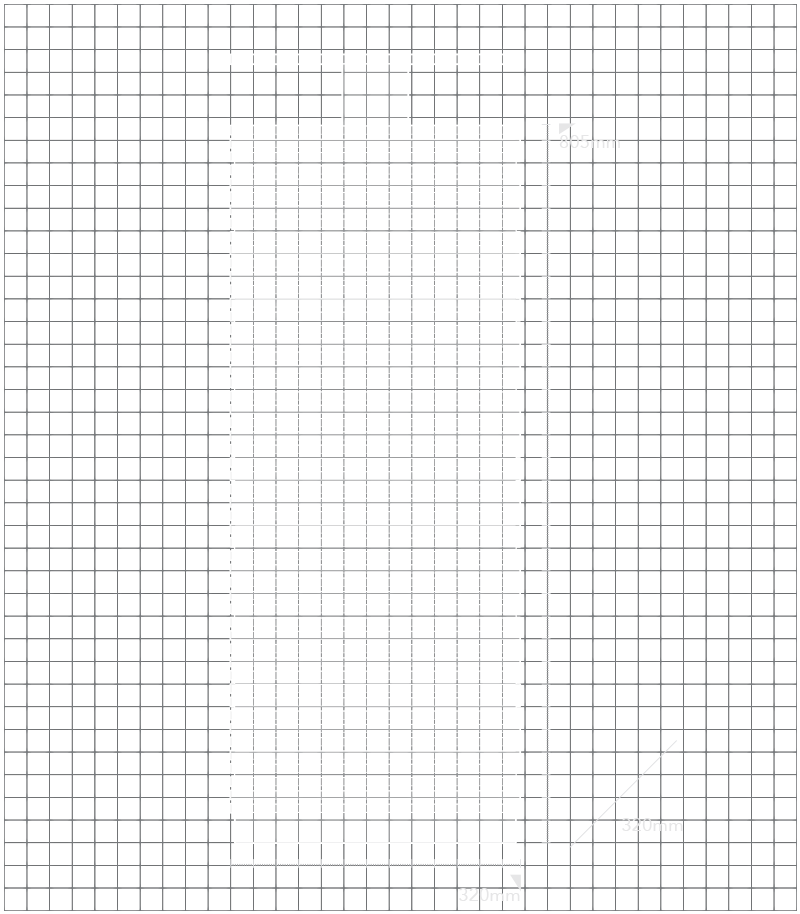
| Ukuran (Lebar x Kedalaman x Tinggi) | 320 mm (W) x 320 mm (D) x 805 mm (H) |
| Berat Bersih | 15 kg |
| Installation Area | Indoor |
Disclaimer:
1. Advanced 6-Step Filtration removes up to 99.999% Ultrafine Dust and 99.9% Airborne viruses, bacteria, black mold.
2. Double HEPA with advanced filter material inactivates up to 99.999% of SARS-CoV-2 Omicron Variant, Influenza A virus (H1N1), Human Coronavirus (HCoV-OC43) with its antibacterial and antifungal performance.
Paket 7 tahun
/Bulan
(Termasuk perawatan dan penggantian filter 7 tahun)
Paket 6 tahun
/Bulan
(Termasuk perawatan dan penggantian filter 6 tahun)
Paket 5 tahun
/Bulan
(Termasuk perawatan dan penggantian filter 5 tahun)
Paket 3 tahun
/Bulan
(Termasuk perawatan dan penggantian filter 3 tahun)
Harga Produk
Filter Package
(Termasuk perawatan dan penggantian filter 1 tahun)